Project Management
Traumatic Brain Injury Drug Treatment (TBI-DT)
Capability Summary:
- Currently there are no Food and Drug Administration (FDA) approved drug therapies for TBI.
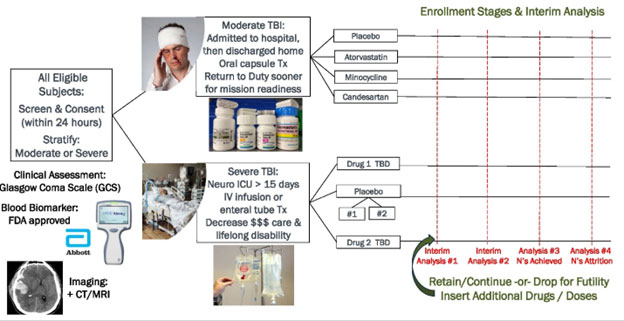
- The TBI-DT program is developing oral, intravenous (IV), and/or intramuscular (IM) drug treatments for TBI: Increment 1 repurposed generic oral drugs for moderate TBI; Increment 2 novel IV drugs for severe TBI.
The FDA-approved, novel or repurposed generic, drug treatment to be administered acutely within 24 hours in the field (and within fixed facilities) to limit brain damage and enhance recovery of Service Members who have experienced a moderate or severe TBI.
Impact: A successful TBI drug treatment will save lives, minimize secondary injury, enable prolonged field care, reduce medical evacuations, and return Service Members to duty sooner improving mission readiness.
Concurrent Phase II Adaptive Platform Trials
Pipeline of Multiple TBI Drug Candidates
Seeking Medium – Large Pharma Interest & Partnerships
Last Modified Date: 01/09/2025













