Project Management
Analyzer Traumatic Brain Injury (ATBI)
Capability Summary:
- The ATBI is Food and Drug Administration (FDA) approved to assess Service Members for TBI biomarkers using only a few drops of blood. Results are obtained in about 15 minutes. Negative results allow providers to rule-out the need for a CT scan.
- In conjunction with other clinical data, this assessment tool helps theater-based medical providers assess, triage, and manage TBI casualties by providing high confidence as an objective TBI assessment tool.
Impact:
- Inform evacuation decisions at Role 2 and 3 locations within the deployed environment, enabling strategic use of potentially limited evacuation assets in Large Scale Combat Operations.
- If available during Counter Insurgency Operations from 2002-2018, the ATBI may have prevented approximately 33% of all rotary-wing evacuations for isolated mild TBI.
To date, the ATBI was utilized in multiple clinical labs within USCENTCOM as part of an operational utilization assessment informing its impact on clinical workflow and operation in the deployed setting. Initial fielding to the Army anticipated to start in fiscal year 2025.
Initial fielding to the Army is anticipated to start in 2025.
TBI Whole Blood Test Process Description:
- Draw venous whole blood from patient sample using a pipette.
- Apply 20µL of patient sample to sample-well on i-STAT TBI cartridge.
- Insert the cartridge into the i-STAT Alinity device and wait for test to complete.
- Retrieve and interpret test results.
TBI Test Results are qualitative, but also include semi-quantitative values. Currently, quantitative values are not FDA approved/cleared to inform patient assessment or care.
- Elevated: Represented by an upwards pointing arrow. Patient sample contains analytes in quantities that are outside the normal range (top, left and right figures).
- Non-Elevated: Patient sample contains analytes in quantities that rule out the need for a head CT (bottom, left and right figures).

TBI Whole Blood Test Process (steps 2 – 4)
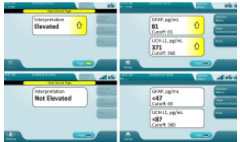
TBI Test Result Screens
Last Modified Date: 01/09/2025













