Blood Products and Components for our Warfighters and Military Working Dogs
Blood products are essential to effective combat casualty care, and early transfusions improve casualty survival rates. Development efforts focus on blood products that can be deployed in austere environments where mobility is required, including cold platelets, freeze-dried plasma and safe whole blood. Our Military Working Dogs (MWD) are critical to saving lives on the battlefield, and they are also at risk for traumatic injuries and hemorrhage. Whole blood is preferred to treat MWDs with severe blood loss, but this is not always available at the point-of-injury. Canine freeze-dried platelets and canine freeze-dried plasma can bridge this gap.
Canine Blood Products
The MWD is a critical member of the tactical or search and rescue teams. Canine blood products such as freeze-dried plasma (CFDP) and platelets (CFDPlt) address the need for treatment of MWDs with severe blood loss close to the point-of-injury in an austere environment, thereby improving patient outcomes.
USAMMDA is managing an effort to assess the value of treatment with CFDP and CFDPlt as part of damage control resuscitation. The goal is to obtain results that assist the development of doctrine that is specifically aimed at saving the lives of wounded military working dogs.
In 2020, a USAMMDA-led team awarded BodeVet, Inc. a contract to perform a clinical study that will involve wounded dogs that have been brought to veterinary treatment facilities. With consent of the owners, dogs that meet enrollment criteria will be entered into the study on a randomized basis to receive the current standard of care, CFDP, CFDPlt, or both CFDP and CFDPlt. The canine trauma study has received regulatory approvals for initiation in April 2021, with completion scheduled for September 2022.
The MDD/MS B brief occurred on June 15, 2020.
Cryopreserved Platelets (CPP)
Platelets are one of the four major components (salt solution, plasma, red blood cells and platelets) necessary for successful treatment of combat casualties with massive hemorrhage on the battlefield. Liquid Stored Platelets (LSP) have a very short shelf-life (seven days or less) once collected, and these are difficult to supply on the battlefield.
Due to the short shelf-life of LSP, approximately 70 percent of LSP produced in theatre are outdated and discarded as waste. Additionally, in-theater walking donor platelet apheresis does not meet the peak demand needs during mass casualty (MASCAL) situations. Only limited stockpiles of platelets are possible with this process. Cryopreserved Platelets (CPP) are human platelets frozen at -80°C in six percent dimethylsulfoxide, a cellular protectant during freezing, and stored at less than or equal to negative 65°C. CPP will have a significantly longer shelf-life (two years or more) compared to LSP, thus eliminating waste and reducing the logistical burden of supplying platelets to the battlefield. CPP will be able to be stockpiled, and these have the potential to eliminate the supply gap, particularly in large-scale emergency scenarios. CPP will be utilized for the treatment of acute hemorrhage in patients with a platelet deficiency or a platelet dysfunction, when liquid stored platelets are unavailable.
Regulatory sponsorship was successfully transferred to Cellphire, Inc. in early 2020. Since then, Cellphire has submitted the annual report to the FDA, establishing the manufacturing process, and also received concurrence on their Phase 2/3 clinical trial design from the FDA. Cellphire, Inc, along with their Contract Research Organization (CRO), can begin activating clinical trial sites to enroll patients necessary for the trial. However, the COVID-19 pandemic may have an impact on schedule as a lot of clinical trials sites are being prioritized for COVID- related trials.
Freeze-Dried Plasma (FDP)
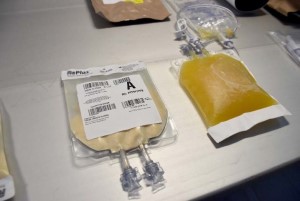
Between 2001 and 2011, up to 26 percent of total pre-Medical Treatment Facility combat deaths may have been potentially survivable. Ninety-one percent of the potentially survivable deaths were due to hemorrhage. Plasma is one of three major components (plasma, red blood cells and platelets) necessary for successful treatment of combat casualties that have experienced massive hemorrhage on the battlefield.
Human plasma, usually fresh frozen plasma (FFP), that has had the water removed, generally by freeze drying (lyophilizing), is commonly called Freeze-Dried Plasma (FDP), or lyophilized human plasma. FDP is a formulation of plasma that enhances logistical support for treatment of combat casualties by reducing freezer requirements typically needed for FFP, and by reducing breakage and waste that has typically been associated with FFP. FDP will reduce waste of plasma by eliminating breakage and outdating of FFP after thawing, and reduce the logistical burden associated with storage requirements for FFP. FDP will permit positioning of plasma forward of ROC 3, potentially saving lives while simultaneously reducing the logistics burden.
The FDP product is currently under development in a collaborative effort between the USAMRDC and Vascular Solutions. (VS, a subsidiary of Teleflex, Inc.), located in Minneapolis, Minnesota. Under Public Law (PL) 115-92 and the Agreement between the FDA and DOD to accelerate the development of critically needed DOD products, the FDA has placed licensure of FDP on fast-track to a Biologics Licensing Agreement (BLA). VSI is currently compiling manufacturing data, to be combined with clinical data generated under a USAMRDC-contract to Westat (a contract research organization), for a final BLA submission targeted for early 2021. VS are also continuing manufacturing development and validation for larger-scale FDP production, which will be used for the Phase 2 trial planned for early 2021 as well as a subsequent pediatric trial. VS assumed full regulatory sponsorship of the product, and is responsible for commercialization and post-licensure requirements for the product.













