USAMMDA Selected to Manage Tier 1 Acquisition Program Funding for Three COVID-19 Response Efforts
Three of the 10 COVID-19 response projects selected by the Defense Health Agency to receive Tier 1 acquisition program investments to support the COVID-19 effort are being managed by the U.S. Army Medical Materiel Development Activity's Warfighter Protection and Acute Care Project Management Office.
The WPAC team assigned to these projects is comprised of military, civilian and contractor personnel who work together to develop and deliver infectious disease drug treatments, vaccines and diagnostics to protect and sustain our nation's Warfighters. The group will use the DHA funding to improve capabilities to detect the COVID virus and the body's response to it, and to develop a candidate treatment.
The Tier 1 acquisition program investments are aimed at supplying testing capabilities that can be rapidly deployed, as well as improvements to existing testing capabilities, vaccines and treatments.
The three WPAC projects selected are the phospholipase A2 Inhibitor (Varespladib) for SARS-CoV2 treatment, the SARS-CoV-2 Rapid Diagnostic Lateral Flow Tests for direct antigen detection and serology, and the BioFire Defense COVID-19 Test 510(k) with Sample Expansion Option.
"Improved detection and drug treatment options are important tools to our Warfighters and the Nation in the fight against SARS-CoV-2. Diagnostics and treatment drugs will help prevent the further spread of SARS-CoV-2 and to potentially save lives of those infected," said Dr. Lawrence Lightner, WPAC Project Manager.
The first project selected is the secreted phospholipase A2, or sPLA2, Inhibitor (Varespladib) for SARS-CoV2 treatment. The sPLA2 Inhibitor is a small-molecule drug that prevents the molecule called phospholipase A2, which is associated with inflammation and other dysregulation and destruction in the lung that is characteristic of Acute Respiratory Distress Syndrome. ARDS is brought on by several diseases, including COVID-19, and leads to degradation of lung function, inadequate oxygen supply and, frequently, death or severe long-term breathing problems. Together with the industry partner, Ophirex, Inc., the WPAC PMO will work to develop Varespladib as an ARDS preventative and treatment in COVID-19 patients. The goal of drug treatment is minimized hospitalization, critical care treatment and survival.

On June 19, the U.S. Army Medical Research Acquisition Activity awarded a $9.9 million contract to Ophirex to manufacture two versions of the drug – a tablet to be taken orally, and an injectable version – to be tested in clinical trials beginning this year. Varespladib is currently being evaluated as a snakebite envenomation treatment and was previously tested against sepsis and acute coronary syndromes; those trials showed it to be safe and well-tolerated in patients.
"This drug doesn't kill SARS-CoV2. Instead, it is anticipated to stop what the virus does to the body – the symptoms of COVID-19 that are killing people and keeping them in the hospital," said Dr. Lindsey Garver, product manager for this effort within the WPAC PMO. "Since it's already been studied for other purposes, we know a lot about its safety profile and can move very quickly to implementation and, hopefully, saving lives."
The second project selected was regulatory progression of BioFire Defense's COVID-19 test. The U.S. Food and Drug Administration authorized this test for Emergency Use on March 23. The test is performed on the BioFire® FilmArray® instrument, present in many Department of Defense facilities. It provides a "detected" or "not detected" report about 50 minutes after a sample, a nasopharyngeal swab in transport medium, is loaded for analysis. While the FDA's Emergency Use Authorization allows this test to be used, the 510(k) route to FDA clearance, which involves the acquisition of additional performance data, will provide additional confidence in the accuracy of test results and support use of the test even after the COVID-19 emergency expires.
The WPAC team worked closely with USAMRAA to finalize BioFire Defense's commitment to perform the required studies, within the specifications of the $3.1 million contract that was awarded June 4.
"We are capitalizing on the work done by our colleagues at JPEO-CBRND [Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense] earlier this year, when they partnered with BioFire Defense to start the development of the COVID-19 test," said Dr. Clifford Snyder, product manager for this effort within the WPAC PMO. "This test detects SARS-CoV-2 nucleic acid with a high degree of specificity and sensitivity. The virus can't hide from this test."
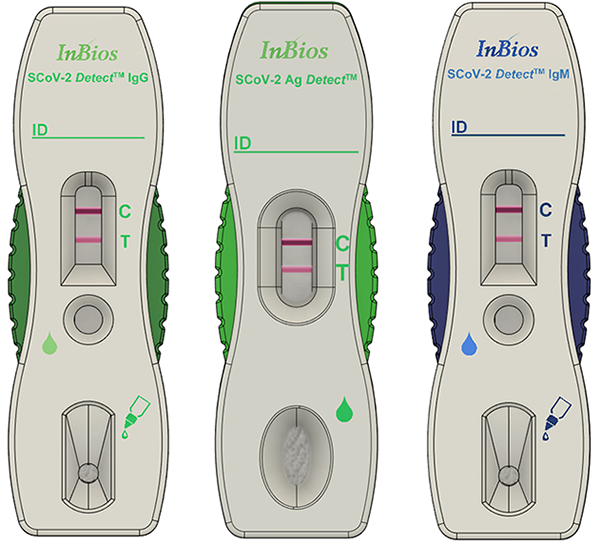
The third and final project selected includes two point-of-care diagnostic tests that will be able to identify persons infected with SARS-CoV-2: the SCoV-2 Ag Detect™ and SCoV-2 Ab Detect™. The SCOV-2 Ag Detect™ is a direct antigen-based test that uses a proprietary "dip-stick"-like test to detect several SARS-CoV-2 antigen targets present in respiratory samples collected by nasopharyngeal swab. The SCoV-2 Ab Detect™ is a serology "dip-stick"-like test that will use blood collected by finger stick to detect SARS-CoV-2-specific immunoglobulin M/immunoglobulin G antibodies in individuals who meet either clinical and/or epidemiological criteria to infer recent or prior infection. Each of these tests will be able to provide results within 15 to 30 minutes of sample collection.
USAMMDA leveraged its existing Indefinite Delivery/Indefinite Quantity contract with InBios International, awarding a task order on June 15 for $11.9 million to support the development of both of these diagnostic tests to achieve EUA and ultimately 510(k) clearance with the FDA. The DHA is funding development activities occurring in fiscal year 2020 to achieve EUA status.
The aim is for improved testing to deliver faster, more accurate detection of SARS-CoV. This can streamline surveillance of virus transmission and thereby minimize time in quarantine, reducing asymptomatic contact, and relieving the burden on testing labs.
The three selected projects represent the DOD's commitment to protect the health and safety of our Warfighters, as well as to leverage our unique capabilities and partnerships that strengthen the "whole-of-government" approach to fighting the worldwide COVID-19 pandemic.
"USAMMDA teams work every day to develop and deliver a broad array of lifesaving medical products for our Warfighters. The WPAC PMO's efforts showcase this breadth and the development of promising technologies to save lives across the nation." said Army Col. Gina Adam, USAMMMDA commander.
The successful development of these important projects is intended to benefit both our military and civilian populations. With a mission to develop and deliver quality medical capabilities to protect, treat and sustain the health of our nation's Service Members, USAMMDA remains focused on providing life-saving drugs and devices that meet or exceed the intended requirements of the DOD. Without question, the men and women of the WPAC PMO team serve to highlight the determination and proficiency of the entire organization.
USAMMDA is a subordinate command of the U.S. Army Medical Research and Development Command, under the Army Futures Command. As the premier developer of world-class military medical capabilities, USAMMDA is responsible for developing and delivering critical products designed to protect and preserve the lives of Warfighters across the globe. USAMRDC is leading research to prevent, detect and treat COVID-19. USAMMDA is applying existing field-leading research capabilities, a global research network and established partnerships to support the Whole-of-Government response to COVID-19.













