Saving Lives with Freeze-Dried Plasma
At the U.S. Army Medical Materiel Development Activity, Fort Detrick, Maryland, the end goal of every effort remains focused on saving lives. With a mission to develop and deliver quality medical capabilities to protect, treat and sustain the health of Service Members worldwide, USAMMDA's successful endeavors result in everything from drugs to vaccines to medical support equipment – and freeze-dried human blood plasma.
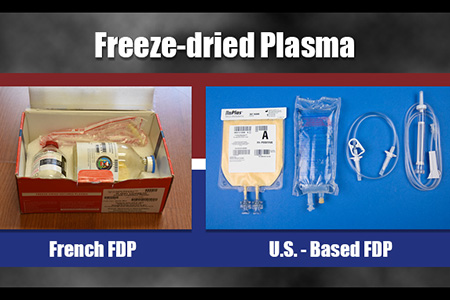
Blood loss from catastrophic injuries is a major factor in battlefield deaths. To ensure that our military medical teams can provide effective far-forward treatment of severe hemorrhage, two separate offices at USAMMDA are dedicated to delivering a freeze-dried plasma (FDP) product to our Warfighters. While the Pharmaceutical Systems Project Management Office works steadily to gain U.S. Food and Drug Administration approval of a new U.S.-based FDP product, USAMMDA's Force Health Protection Division manages the current distribution and use of a French FDP product under a stringent "expanded access" Investigational New Drug treatment protocol by the FDA, which allows for the closely monitored application of certain investigational products not being used in clinical trials.
The primary advantage of using freeze-dried blood plasma is that it is a stable, dry product that remains effective at room temperature for up to two years, until it is reconstituted with sterile water when ready to be used by medical personnel. Unlike fresh, frozen plasma, which requires refrigeration at all times, FDP can be stored practically anywhere, and transported in backpacks to treat wounded Warfighters in the field.
Both plasma products remain extremely important to the health and readiness of Special Operations Forces in all four U.S. military branches: Army, Navy, Air Force and Marines. However, the French FDP is being used primarily as a stop-gap measure until the U.S. product is approved for use. The bottom line of these two separate efforts is to ensure the U.S. Special Operations Command has an effective FDP product in place, at all times, to help save the lives of SOF Service Members across the globe and foreign nationals supporting them.
And, fortunately, this has been the case for the better part of the last decade.
Developing a U.S.-based FDP product
Since the beginning of this critical effort, the PSPMO's Andrew Atkinson has served as the product manager overseeing the development of U.S.-based FDP. During our meeting last year, Atkinson said the goal was to have a U.S. FDP product approved and readily available by 2020 – and, currently, this remains the target date.
"We're still holding to the 2020 date," said Atkinson, "but this will depend, in part, on the FDA accepting our clinical development plan. In our pre-IND meeting, the FDA accepted our plan preliminarily, but final approval of the plan will depend on both our clinical safety data and product characterization data. However, we are looking to accelerate the schedule without sacrificing safety and efficacy."
The Department of Defense is funding the development of this new U.S.-based FDP product for many reasons, although most pertain to logistics and availability. The current French FDP product is held in glass bottles with a unique vacuum pressure system to reconstitute the plasma. However, the bottles are a bit heavy and prone to damage if not handled correctly, and the vacuum system requires a spiking process that can be problematic if not done precisely. The new U.S. version will utilize state-of-the-art, lighter plastic bags that use a standard fluid transfer set to mix the product with sterile water for injection, and a standard blood set to direct the plasma into the patient, which Atkinson believes is more efficient for medical personnel.
From a clinical standpoint, the current U.S. study is still on track as the team heads towards completion of the Phase 1 study, which is considering the safety of both the product and process.
"We've finished the first of three cohorts so far, which was about three to four months long," said Atkinson. "There are eight subjects in each cohort of this autologous study, which means that all 24 subjects will receive their own collected FDP."
"Cohort 3 will be a bit longer, about six to seven months," he added, "because the subjects will receive three units of their own fresh frozen plasma, and then three units of FDP, so that we can collect data to check for differences in the various clinical parameters."
The Phase 2 study will look at both the safety and efficacy of the product and process, and Atkinson believes this will begin in late 2018 if all goes well with FDA acceptance of their clinical development plan. He also said that his team is making great progress on utilizing larger, commercial-size freeze-drying machines that can handle up to 500 FDP units per batch, versus a batch size of 25-40 in the clinical-scale machines.
When asked about next steps in the approval process, Atkinson said that USAMMDA's commercial partner is already developing a next-generation plastic bag that will be even more efficient than the one currently being used in clinical trials.
"The new bag will be slightly longer, with a dual permeable membrane system that increases the rate of transfer of moisture from the interior, which will minimize the amount of air bubbles and increase the integrity of the bag," he explained. "All of this will actually help to decrease the cycle processing time from 11 days to seven days, which will speed up the turnaround time for our medical personnel."
Atkinson also clarified that the standard fluid transfer set, the IV blood set and the sterile water for injection have already received FDA approval, so only the plasma product and the bag system are awaiting final approval for use.
While he said that both FDP products are very valuable and help to save lives, Atkinson explained that a U.S.-based product may be a better choice due to the quantity of product that can be produced here at home.
"Both products are made from volunteer donors: the French use French donors and we use American donors for our U.S. FDP," he said. "However, at VSI's production facility, we will have the ability to produce about 800 to 1,000 units per week, whereas the French can supply us with only 2,800 to 3,000 units per year, which is quite a big difference."
But despite the differences between both products, Atkinson made clear that these are not competing efforts, they are complementary.
"The French FDP product is helping to save lives until our U.S. product is approved," he said.
Using French FDP until a U.S.-based FDP is approved
In November 2011, after an urgent request from the USSOCOM commander, the U.S. Army Special Operations Command became the first U.S. military service to use French FDP to treat Warfighters in far-forward locations. Although U.S. military forces had used an FDP product until shortly after World War II, this was halted due to the transmission of blood-borne pathogens. However, the need was present once again, and a new product manufactured by the French military was available. This time, the blood plasma was being purified through a pathogen-reduction system, and the success of the product was evident.
After five years of use within USASOC, the protocol was extended to include three other service component commands of USSOCOM. In August 2016, the U.S. Marine Corp Forces Special Operations Command began using French FDP, and the Naval Special Warfare Command and Air Force Special Operations Command followed in April 2017 and May 2017, respectively. In fiscal year 2017, 430 units of French FDP have been requested by USSOCOM and distributed to the four SOF service components.
Danny Hassan is the FHP Division product manager responsible for overseeing the distribution and use of French FDP throughout USSOCOM.
"My role in FHP is to serve as a conduit between all four [USSOCOM service components] and our regulatory offices here at USAMMDA and USAMRMC [U.S. Army Medical Research and Materiel Command]," he said. "I coordinate to ensure we successfully execute the IND according to the protocol, and I assist with accountability and help to resolve any issues."
Hassan is very familiar with the importance of this product. As a 22-year Army veteran, he spent much of his military career as a combat medic, and during a deployment to Iraq he witnessed the severe injuries caused by improvised explosive devices. He knows the importance of FDP in treating trauma cases on the battlefield, and he has numbers to prove it.
Yes, FDP is still saving lives.
Since its approval for use in 2011, French FDP has been used to treat 24 patients for life-threatening injuries in austere environments, and 17 have survived to transfer of care.
"Without question, the French FDP is helping to save many lives, and we will continue to use this product as long as it's doing what it should, without any adverse effects," he said. "The plasma is leukocyte-depleted and pathogen-reduced, which helps to make sure it is cleaned properly, so I don't foresee any changes to this particular French product. They have been using it for decades with much success."
Hassan said that French FDP is a critical component of the Tactical Combat Casualty Care strategy for hemorrhagic resuscitation in the absence of whole blood. He stressed that providers at the point-of-need have taken experience and medical evidence since Vietnam up to recent conflicts in Afghanistan and Iraq to change the standard of care regarding the use of FDP, and U.S. SOF Service Members have benefitted with increased survival rates.
Hassan's involvement with FHP and USSOCOM is certainly enhanced by his military background. Although he has been with FHP for only five months, he already has been able to help quite a bit in a number of situations.
"Because I was an Army medic, I think this helped in establishing a good rapport right away," he explained. "I understand the field, and we speak the same language, so it was a good fit, which made for a smooth transition."
Saving lives far into the future
Although French FDP is being used only by trained U.S. SOF at this time, once the U.S.-based FDP product gains FDA approval, it may be accessible by a larger number of military medical personnel throughout the world.
So, most would agree that FDP is certainly a very useful, and very important, product.
"The beauty of using FDP is that you can reconstitute it in under two minutes," said Atkinson. "Fresh, frozen plasma must be stored frozen, and takes about 30 to 40 minutes to thaw out. But you still can use both for particular applications – we can use FDP for immediate care, especially in far-forward locations, and then thaw out additional fresh, frozen plasma to use in the same patient later, once they are transported to the medical facility."
As evidenced by the dedication and focus of the USAMMDA team members involved in these two critical efforts, maintaining an effective FDP product for the health, welfare and readiness of our nation's Warfighters will remain a top priority of Army Medicine for years to come. The men and women of our military forces can rest assured that, each day, there are men and women working to ensure they receive the medical products they need, whenever they need them.













